Oocytes and Ovaries
Our research focusses on understanding the intricate and complex mechanisms that regulate oocyte and follicle development in health and disease. The overarching goal is to develop new fertility treatments and fertility preservation methods for girls and women.

WHAT WE DO
The research carried out by the Williams group is to understand ovarian function in health and disease with the aim of developing novel fertility preservation technologies. Under this umbrella there are multiple lines of research including investigating ovarian ageing, follicle selection, primary ovarian insufficiency (POI; previously known as premature ovarian failure, POF), fertility preservation strategies for both women and rhinos.
Unravelling the mechanisms that Regulate Follicle Development
The mechanisms that regulate follicle development are complex and although we know a great deal about the interactions required for successful follicle development, there is still much to be understood about follicle selection and function - particularly regarding the role of the oocyte.
- A mouse model generated by Prof. Williams has been particularly useful in teasing apart the role of the oocyte in follicle development since this model generates oocytes lacking core 1-derived O-glycans and this promotes follicle development leading to an increase in the number of follicles and also ovulation rate. In addition, we have identified a novel role for the oocyte in follicle basal lamina development with follicles containing mutant oocytes having altered basal lamina morphologies, how this affects follicle function is under investigation.
- In addition we also use various in vitro culture systems to investigate follicle culture, notably the reaggregated ovary model, where the ovary is taken apart into single cells and the different populations can be recombined in different ways to answer specific biological questions.

Investigating the aetiology of premature ovarian failure
Premature ovarian failure (POF) is a common condition affecting 1% of women under 40 years of age. However, despite its high prevalence, the majority of the causes for this distressing condition, ~70%, remain unknown. The common perception is that ovaries of women with POF no longer contain follicles, however this is not the case since around 50% of ovaries still contain follicles, and thus the ovaries have stopped functioning, this is also known as resistant ovary syndrome (ROS). Prof. Williams generated a mouse model of premature ovarian failure in order to study the aetiology of this disease with the aim of developing treatments.
The effects of ageing on oocyte quality and follicle development
It is well know that oocyte quality declines with age and the defects that occur are well characterised, however the mechanisms that regulate this declining quality are less well understood and is an aspect of ageing we are investigating. 
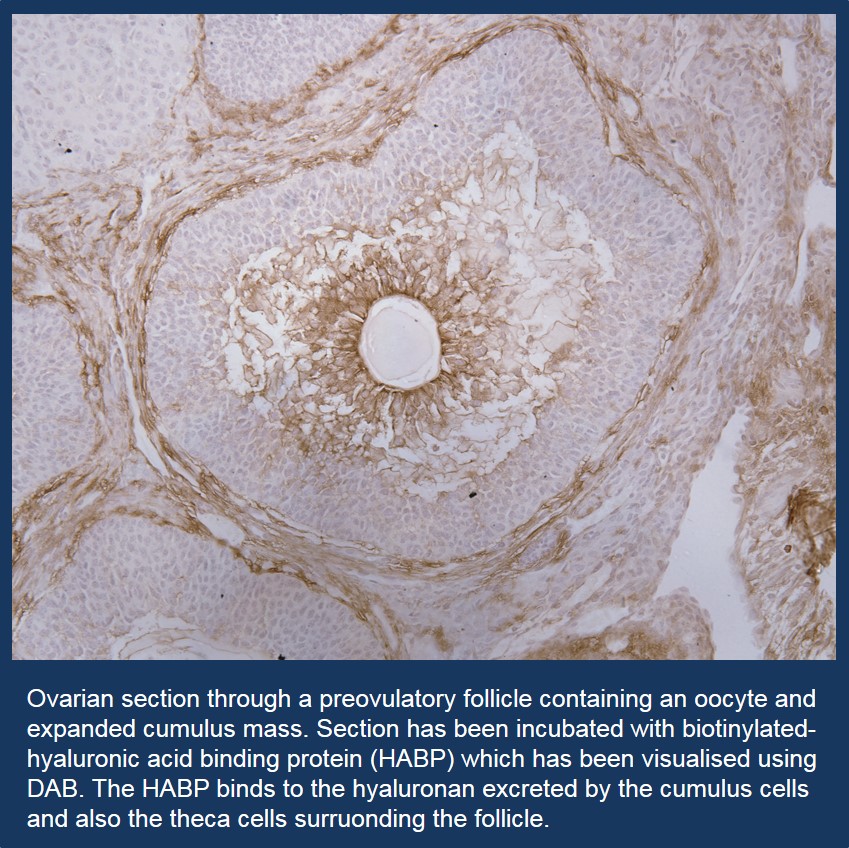
Cumulus Expansion Form and Function
The cumulus mass surrounding the oocyte is required for oocyte maturation and collection of the ovulated egg by the oviduct. Correct expansion requires cumulus cells to secrete extracellular molecules such as hyaluronan, PTX3, TSG-6 which cross-link with the heavy chains (HCs) of serum-derived inter-α-inhibitor proteins resulting in expansion of the cells by mucification. Furthermore, it was previously understood that the relationships between these molecules was tightly linked and all were required as deficiencies in any one of these led to defective expansion and reduced fertility. However, recent studies in our group led by Panayiota Ploutarchou have demonstrated that this perception of cumulus expansion is not strictly true. Investigations into cumulus expansion using the C1galt1 mouse model which ovulates eggs with a modified modified cumulus expansion that does not negatively affect fertilisation or fertility led us to explore this concept by investigating the levels of the different molecules in individual cumulus matrixes. These studies revealed that there is a high degree of flexibility in the levels of the molecules required for cumulus expansion and most surprisingly, considering the perception that the molecules were highly dependent on each other, there was no correlation between the molecules or the activation of the signalling pathways required for expansion.